The high-frequency electrosurgical generator is an electrosurgical instrument that replaces the mechanical scalpel for tissue cutting. The high-frequency high-voltage current generated by the tip of the electrode contacts the body to heat the tissue and realizes the separation and coagulation of the body tissue for the purpose of cutting and hemostasis. It features advantages such as fast cutting speed, good hemostasis effect, improved operation efficiency, and a wide application range.
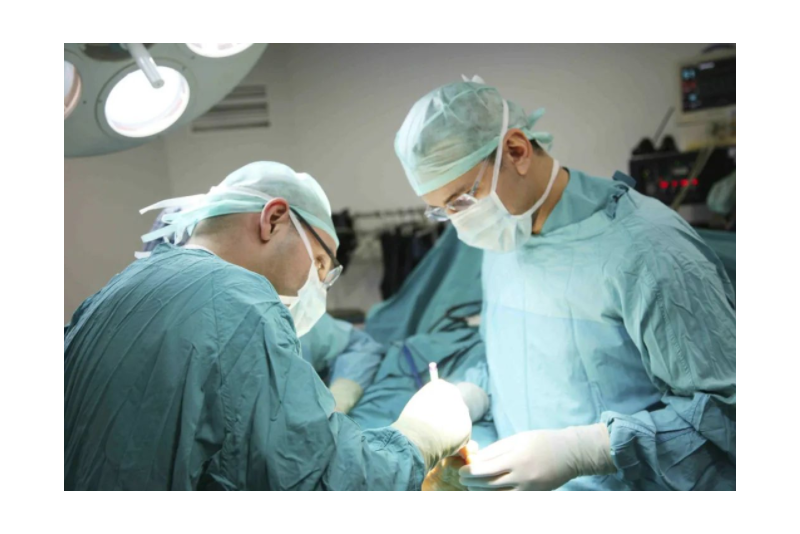
At present, high-frequency electrosurgical generators are widely used in surgical operations in major hospitals across the country, but they are also equipment with higher risk levels. The quality control and performance testing of ESU should not be ignored. Therefore, it is recommended to conduct a complete overhaul of the ESU on a regular basis to ensure its performance and stability.
In the specific practice of high-frequency electrosurgical generator testing, the environment has a significant impact on the testing results. Therefore, it is necessary to control the environment to meet the specific requirements of the testing that the temperature should be controlled at about 15~35 ℃, the relative humidity of the area is less than 80%, the atmospheric pressure of the testing environment is maintained at 86~106 kPa, and the testing area should try to avoid mechanical vibration or electromagnetic interference that affects the normal operation of the verification system.
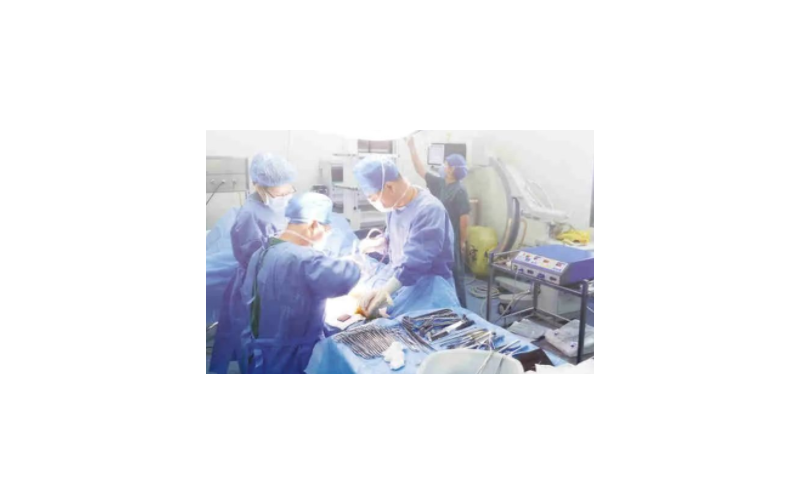
The work scene of high-frequency ESU
Determining the frequency of inspections can make the quality control methodical and planned, and facilitate the allocation and management of personnel resources. In June 2020, the National Medical Products Administration issued the “Notice on Issuing the Standards for Writing Regular Risk Evaluation Reports for Medical Devices”, which provides systematic and standardized guidance on the regular risk assessment of medical equipment, and stipulates that the medical devices that have been approved for registration or filing for the first time shall be completed the regular product risk evaluation report of the previous year within 60 days after every 1 year. Under normal circumstances, the ESU manufacturer also recommends a preventive maintenance test every six months to ensure the performance of the equipment.
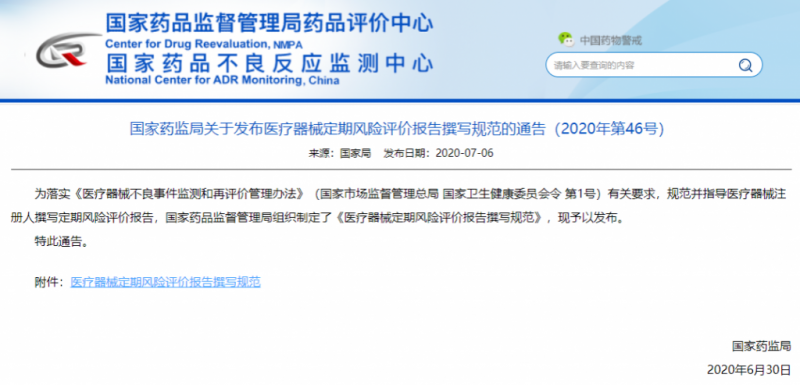
Announcement on the standardization of writing regular risk assessment reports for medical devices
Appearance: In addition to checking the name, manufacturer, specification, model, production number and production information of the equipment, it is also necessary to check the appearance of the equipment to determine whether the equipment is complete, whether the keys are flexible, and whether various touch switches are operable.
Output Power:
The output power test is the central content of the specific test, including many items, such as the measurement of the rated load unipolar output power. During the measurement process, the circuit connection method needs to be checked to determine whether the connection is intact. The specific power is needed to determine whether the error is within the standard range.
For the detection of the unipolar output power of different loads, the specific requirements for the error value are different under different load environments, so the error calculation should be based on the standard to realize the detection.
For the detection of bipolar output power, the specific error should be calculated after the completion of the circuit connection mode to determine whether it meets the requirements.
High Frequency Leakage Detection:
Unipolar loading surgical electrode: It can detect the connection of the circuit, then adjust the specific content in the system, and record the relevant content, and detect the specific value.
Unipolar loading neutral electrode: mainly use equipment to detect specific values in the connected circuit to obtain the detection result.
Unipolar no-load surgical electrode: detect the corresponding connection circuit, and continue to adjust the specific content of the circuit to make it in a standard state to obtain accurate detection data.
Safety Alarm: During the test process, it is necessary to disconnect the connection between the load and the high-frequency electrosurgical generator, and observe whether it alarms in this state.
CF: The connection mode of the circuit for measuring CF is consistent with the connection mode of the measured power. In single-pole measurement, set the rated output power of the tested equipment to be 100W during both cutting and coagulation and the non-inductive resistance to 300Ω.
Ω: In the bipolar measurement, set the rated output power to 50W and the non-inductive resistance to 100Ω; after setting, the electromagnet is excited and the data is recorded to obtain the specific CF value.

EB05 Electrosurgical Generator
EB05 high-frequency electrosurgical unit can be linked with an argon device and can be upgraded to an argon knife to perform APC surgery to meet the needs of general surgery, hepatobiliary surgery and other open surgery and digestive endoscopy for rapid and large-area hemostasis.
All floating output, equipped with instant fault alarm function, the contact area of the plate drops to a dangerous level, and the host immediately cuts off the output, making the clinic more at ease.
Features of EB05
 沪公网安备31011802003750号
沪公网安备31011802003750号