BACKGROUND
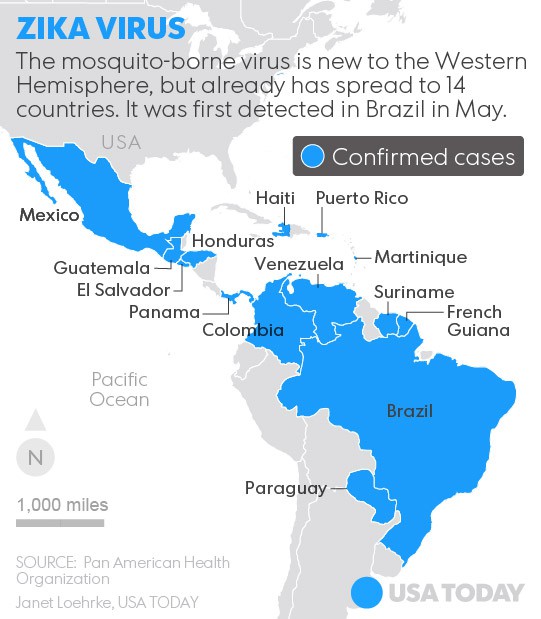
Zika virus (ZIKV), a mosquito borne flavivirus is a pathogen affecting humans in Asia and Africa. Recent outbreaks of Zika virus fever in different regions of the world demonstrate the potential for the arbovirus to spread through territories where the (Aedes) vector is found.
Serological test (ELISA or Immunofluorescence)
ZIKV infection diagnosis relies on serology–which is challenging due to cross-reactions with other flaviviruses and/or absence or low titer of IgM and IgG antibodies at early phase of infection- virus isolation, which is labor intensive, time consuming and requires appropriate containment.

Molecular diagnostic (Conventional or real-time RT-PCR)
During the first 5 days after the onset of clinical picture, viral Ribonucleic Acid (RNA) can be detected in serum by molecular techniques (conventional or real-time RT-PCR). Real-time RT-PCR (rRT-PCR) is an appealing option as a rapid, sensitive and specific method targeting the envelope gene or NS5 region

Experimental design for Real-time RT-PCR
Viral RNA is extracted from clinical specimens using RNA Viral Kit. RNA is amplified by Real-time PCR in a Heal Force X960 Real-Time cycler. One-Step RT-PCR kit mixture includes enzyme mixture (reverse trancriptase RT and Taq polymerase), buffer, primer, probe, DNA RNA free water. Each amplification run contained one negative and one positive control. The negative control consisted of blank reagent and water. For the positive control, nucleic acid extracted from virus stocks was used. The following thermal profile was used a single cycle of reverse transcription for 10 min at 50°C, 15 min at 95°C for reverse transcriptase inactivation and DNA polymerase activation followed by 40 amplification cycles of 15 sec at 95°C and 1 min 60°C (annealing-extension step). The data were analyzed using the real-time PCR software from Heal Force.
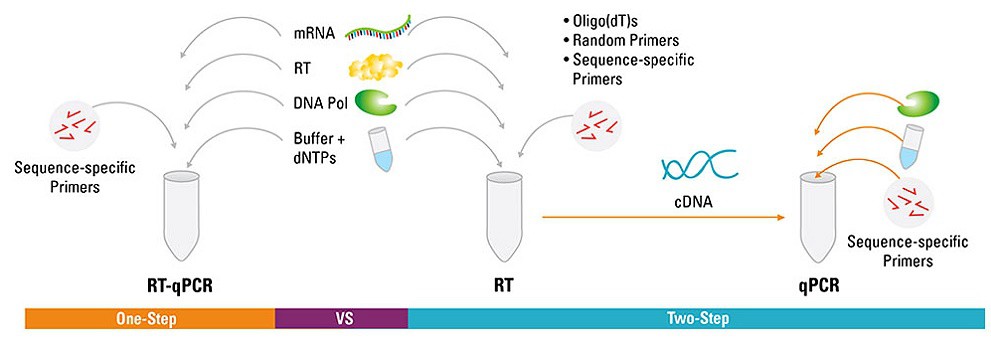
Why One-step qRT-PCR ?
In a one-step reaction, the reverse transcriptase and thermostable DNA polymerase are both present during reverse transcription, and the RT is inactivated in the high-temperature DNA polymerase activation stage (the so-called hot start). Normally, the RT is favored by a buffer that is not optimal for the DNA polymerase. Thus, one-step buffers are a compromise solution that provide acceptable but not optimal functionality of both enzymes. This slightly lower functionality is compensated by the fact that, using this single-tube procedure, all cDNA produced is amplified in the PCR stage.
The benefits of one-step qRT-PCR include the following:
• Contamination prevention—the closed-tube system prevents introduction of contaminants between the RT and PCR stages
• Convenience—the number of pipetting steps is reduced and hands-on time is minimized
• High-throughput sample screening—for the reasons mentioned above
• Sensitivity—one-step reactions may be more sensitive than two-step reactions because all the first-strand cDNA created is available for real-time PCR amplification
 沪公网安备31011802003750号
沪公网安备31011802003750号